What Happens When Your Gut and Brain Lose Connection?
Imagine your gut and brain as two bustling cities, deeply connected by an intricate highway system. Messages, resources, and even emergency signals travel between these hubs, ensuring harmony. However, when traffic jams or hostile takeovers disrupt communication, chaos ensues.
This is the story of the gut-brain axis—a bidirectional communication system that plays a crucial role in neurological health and disease. As science uncovers more about this delicate balance, could the secret to treating diseases like Alzheimer’s and Parkinson’s lie in restoring this connection?
The Highway of Communication: How Does Your Gut “Talk” to Your Brain?
The gut-brain axis consists of neural, hormonal, and immune pathways. But what’s really happening beneath the surface?
The gut-brain axis consists of neural, hormonal, and immune pathways. Like an advanced transport network, the vagus nerve acts as an expressway, transmitting signals between the gut and brain. Meanwhile, cytokines and neurotransmitters function like messengers, carrying vital information. When everything runs smoothly, these cities thrive. But when disruptions occur—such as in neuroinflammatory conditions—the result can be traffic congestion, sending stress signals that exacerbate disease.
Recent research reveals that bacterial molecular pathways and metabolites from the gut microbiota play significant roles in both protecting and driving the development of neurodegenerative diseases. Pro-inflammatory and anti-inflammatory microbes are the two main players in this microbial world, with the balance between them crucial for the health of the gut-brain axis. When this balance is disturbed, dysbiosis—an imbalance of gut microbes—can trigger systemic inflammation and impact brain function.
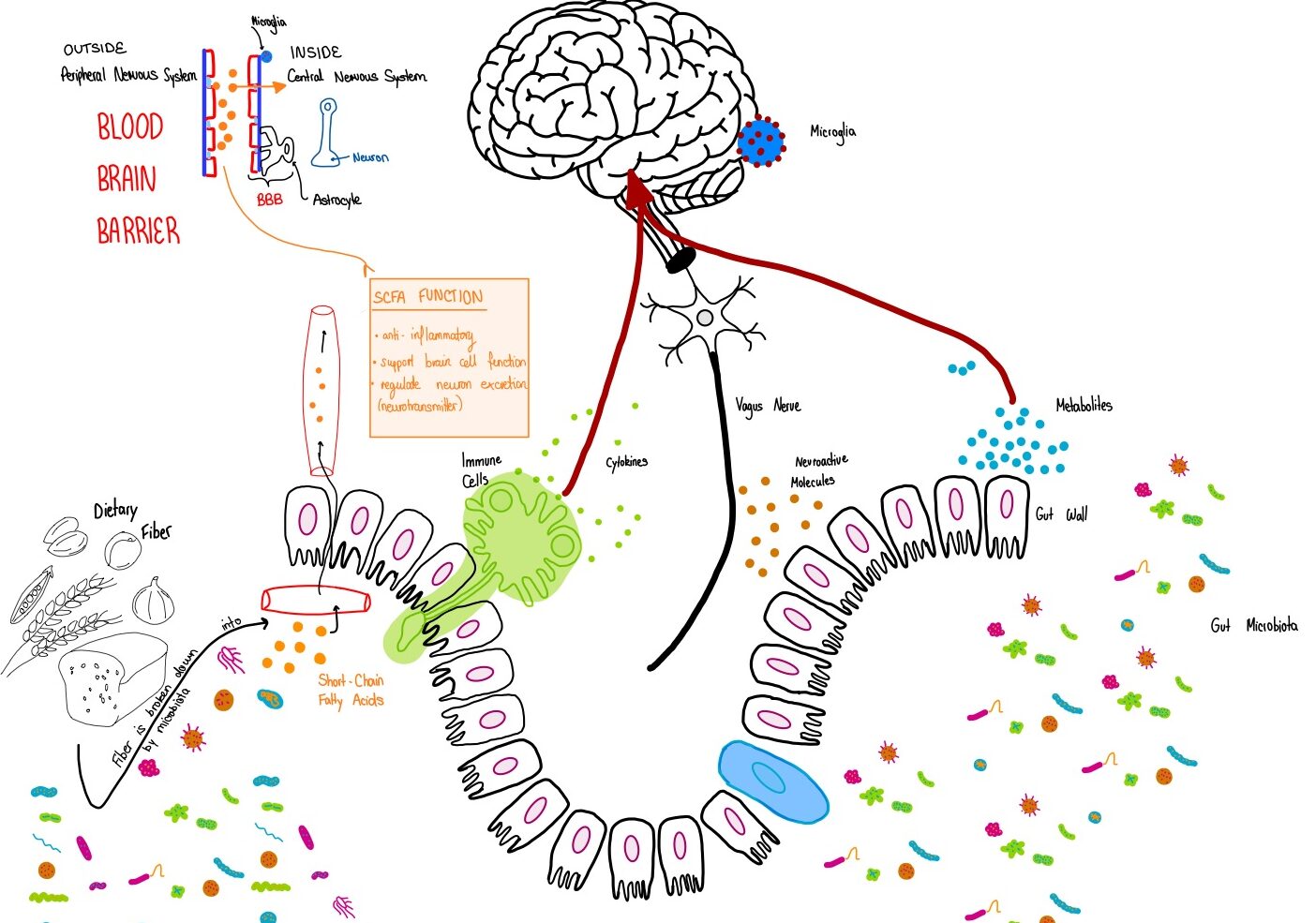
When sodium butyrate is in short supply, it’s like there aren’t enough repair workers in the city, and the gut’s protective walls weaken. Harmful substances—like toxins or bad bacteria—can then slip into the bloodstream and reach the brain, triggering microglia (the brain’s immune cells) and causing inflammation. This can lead to neurological issues like cognitive decline and mood disorders. Thus, sodium butyrate and other SCFAs play a vital role in keeping the gut city’s infrastructure intact and in supporting healthy communication with the brain.
The Brain’s Defense Force: Are Microglia Protecting or Attacking Your Brain?
In the brain city, microglia serve as the local law enforcement. They exist in two main forms: M1, “the Defenders” that fight off invaders but can cause collateral damage, and M2, the “Peacekeepers” that repair and restore balance. In a healthy state, these forces operate in harmony. However, gut disturbances can send distress signals, pushing microglia into an overactive M1 state, fueling chronic inflammation and neurodegeneration.
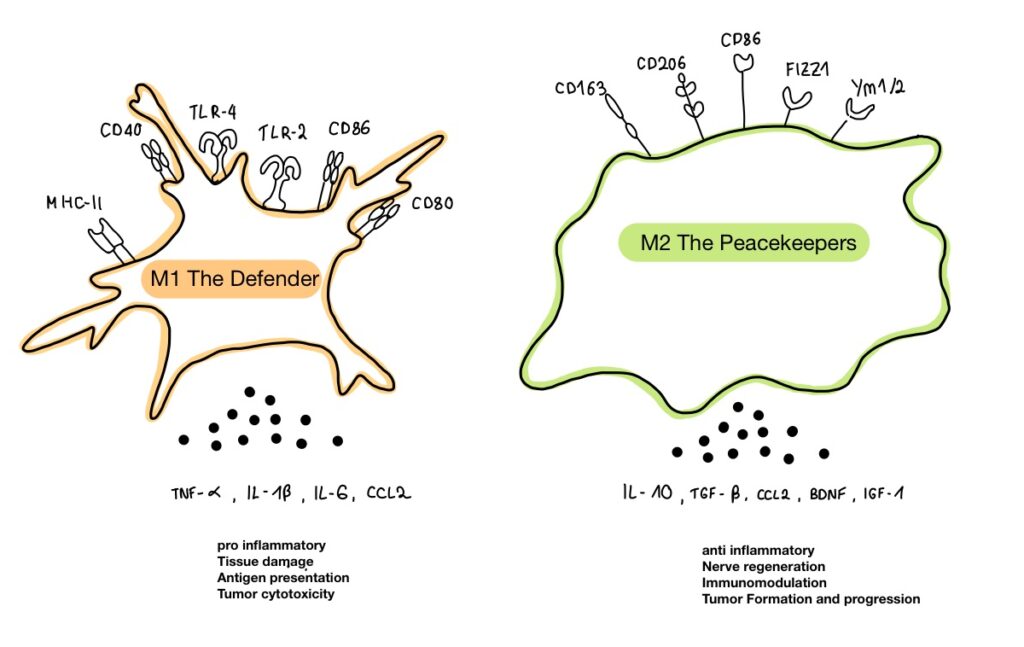
Microglia are the brain’s primary immune cells, and they play a dual role in both injury response and repair. When the brain faces a threat, microglia switch to an aggressive M1 state, unleashing pro-inflammatory cytokines to fight the damage. However, when healing is needed, microglia shift to the M2 state, producing anti-inflammatory cytokines that aid recovery. This balance is crucial for the brain’s ability to respond to injury while preventing chronic inflammation.
New research has found that microglial activation plays a critical role in post-cardiac arrest (CA) syndrome (PCAS), which is common among survivors of out-of-hospital cardiac arrest (OHCA). Cardiac arrest (CA) occurs when the heart suddenly stops beating, cutting off blood flow to vital organs, including the brain. The brain is particularly vulnerable to ischemia (lack of oxygen) and inflammation, which are major causes of death and long-term disability in cardiac arrest patients. Interestingly, microglia are involved in the inflammatory response that follows brain injury due to cardiac arrest, and their activation can make the situation worse by amplifying neuroinflammation.
During these episodes, a key player in the brain’s immune response is the Toll-like receptor 4 (TLR4), which is involved in recognizing harmful stimuli and triggering inflammation. After a cardiac arrest, the activation of TLR4 on microglia leads to the release of inflammatory molecules, further damaging the brain. Studies have shown that reducing this activation could help protect the brain. This is where the gut-brain axis comes into play—microglia’s behavior can be influenced by the gut microbiome, and short-chain fatty acids (like sodium butyrate) produced by beneficial gut bacteria can shift microglia from their harmful M1 state to the protective M2 state, reducing neuroinflammation and improving recovery.
One promising finding comes from studies using sodium butyrate (SB), a short-chain fatty acid produced by the gut microbiota. SB has been shown to help reduce inflammation in the brain by modulating microglial activation. It does so by promoting the shift from the M1 (inflammatory) state to the M2 (protective) state. In models of cardiac arrest, SB has been found to improve neurological outcomes by regulating microglial activity and reducing the harmful effects of inflammation.
The research also emphasizes the connection between gut health and brain injury in cardiac arrest survivors. Bacterial imbalance in the gut—dysbiosis—can worsen brain injury after resuscitation (process of reviving someone from unconsciousness), partly due to the gut’s role in triggering inflammation and affecting microglial function. These findings suggest that restoring a healthy gut microbiome could be a potential therapeutic strategy for improving brain recovery after cardiac arrest.
The Seamless-Walk between two Cities: Practical Steps for a Healthy Gut-Brain Axis
Given the recent findings on the gut-brain connection, several promising therapeutic avenues are being explored to restore microbial balance and vascular integrity. One such strategy is microbiota-based therapy, aimed at restoring protective microbial taxa to maintain intestinal barrier function and reduce inflammation. This includes interventions like probiotics, prebiotics, and dietary adjustments that support the growth of beneficial microbes, such as butyrate-producing bacteria (butyrate = short-chain fatty acid (SCFA)), which help promote anti-inflammatory pathways in the gut-brain axis.
📖 Step-by-Step “Recipe”
To maintain harmony between the brain and gut, think of it as a “seamless walk between the two cities.” Here’s your step-by-step “recipe” for supporting the smooth flow of communication and well-being:
- Fiber-Rich Diet:
- fuels your gut’s microbial residents, promoting a healthy, diverse microbiome
- microbes break down fiber into microbial metabolites (like short-chain fatty acids or SCFAs), which help reduce inflammation
- encourages the growth of beneficial bacteria, like butyrate-producing microbes, which play a key role in maintaining intestinal barrier function
- fuels your gut’s microbial residents, promoting a healthy, diverse microbiome
- Probiotics & Prebiotics:
- Prebiotics are nutrients that feed the beneficial bacteria in your gut, while probiotics are the good bacteria themselves
- Together, they help maintain a balanced microbiome, support intestinal barrier integrity, and boost immune responses, ensuring smooth communication between the two cities
- Stress Management:
- Chronic stress can disrupt the gut microbiome and send stress signals to the brain, worsening inflammation
- Exercise:
- Physical activity supports brain function while also promoting gut health by reducing inflammation and supporting microbial diversity
- Phytonutrients:
- Found in plant-based foods, phytonutrients are natural compounds that protect your cells, reduce inflammation
- Postbiotics:
- These are beneficial by-products produced by probiotic bacteria. They help strengthen the immune system, reduce inflammation, and ensure both the gut and brain remain in harmony
As research progresses, understanding how these bioactive compounds function will be essential for developing personalized nutritional interventions and therapies for neurological disorders. By following these steps, you can help restore balance, promoting a healthy gut-brain connection, and ensuring both cities thrive together.
Conclusion: How Small Changes Can Empower You to Take Control of Your Neurological Health
What if the key to a healthier brain and body is within your reach right now?
As research continues to reveal the intricate dance between the gut and the brain, it becomes increasingly clear that the power to influence our neurological health lies in our hands—often through the smallest of daily choices. We now understand that maintaining microbial balance is not just about digestion; it’s about our mental well-being, our emotional resilience, and even our ability to ward off chronic diseases.
Small, intentional changes, such as adjusting your diet or managing stress, can have profound effects, reaching deeper into the physical and psychological realms than we may realize. The gut-brain axis shows us that our bodies are not passive recipients of external forces but active participants in their own health. The food we eat, the habits we form, and the mindset we cultivate can shape our brain’s function and even its response to disease.
While the science behind this connection is still unfolding, there’s no doubt that everyday actions—like choosing the right foods or incorporating mindfulness—are stepping stones toward a healthier, more balanced life. These actions allow us to take control, making informed decisions that support the vitality of both our gut and our brain. The journey to better neurological health is not distant or abstract; it starts with the choices we make today. By nurturing the connection between these two vital cities, we are paving the way for a healthier, more empowered future.
📚Further Reading
Here are some recent studies and articles that delve into the gut-brain axis:
The Gut-Brain Axis Study
This research project, conducted with 250 healthy male volunteers, explores the connection between the gut microbiome and brain function. Its aim is to provide deeper insights into how our gut health can shape cognitive abilities and behavior.
Read more: University of Reading Research2. Microbiota–Gut–Brain Axis and Its Therapeutic Applications in Neurodegenerative Diseases
This review discusses how the microbiota-gut-brain axis regulates glial functions and its potential as a therapeutic target for neurodegenerative diseases like Alzheimer’s and Parkinson’s.
Read more: Nature3. Gut-Brain Axis and Neuroplasticity in Health and Disease: A Systematic Review
Examining current evidence, this review highlights how the gut-brain axis influences brain connectivity and neuroplasticity, emphasizing its significant role in both health and disease.
Read more: Springer4. The Microbiota–Gut–Brain Axis and Neurodevelopmental Disorders
This article explores the impact of gut microbiota on central nervous system processes and neurodevelopment, with significant implications for understanding and treating neurodevelopmental disorders.
Read more: Oxford Academic5. The Gut Microbiota-Brain Axis Role in Neurodegenerative Diseases
This review updates our understanding of the microbiota-gut-brain axis and its role in neurodegenerative diseases, offering potential new therapeutic strategies.
Read more: Open Research Europe6. Diet Tweaks to Improve Mental Health: Insights from a Leading Scientist
Professor Valerie Taylor shares research-backed dietary changes that could improve gut health, ultimately benefiting mental well-being.
Read more: Business Insider




