Dynamic Homeostasis – Persistence from Thermodynamic Equilibrium
Under normal physiological conditions, cells maintain a finely tuned spatial configuration of organelles, signaling hubs, and molecular gradients. This order is not static: it is actively sustained through continuous energy expenditure, primarily via the cytoskeletal network. In this state of dynamic homeostasis, the cell resists spatial entropy by channeling ATP into filament turnover, intracellular transport, and mechanical tension regulation.
The Cytoskeleton as a Spatial Entropy Management System
The cytoskeleton serves as the primary infrastructure for spatial order in the cell. Composed of microtubules, actin filaments, and intermediate filaments, this network dynamically organizes intracellular components against the natural tendency toward diffusion and disorganization.
- Microtubules form polarized tracks that span the cytoplasm, guiding vesicles, RNAs, and organelles along motor-driven routes.
- Actin filaments, particularly enriched at the cell cortex, regulate local membrane dynamics, cortical tension, and endocytosis.
- Motor proteins (e.g., kinesin, dynein, myosin) convert chemical energy into directed movement, ensuring cargoes reach precise destinations.
This activity functions as an entropy-dissipating mechanism: energy is used not to build permanent structure, but to preserve coherent, functional geometry in a fluctuating environment.
Vale (2003) showed that the intracellular transport system reduces the entropic cost of random diffusion by directing cargo flow, creating low-entropy spatial distributions essential for function. Theriot (2000) demonstrated that actin treadmilling is not a passive cycle but a highly regulated ATP-dependent process, enabling dynamic yet stable polarity at the cell front.
Energy Flow and Spatial Patterning
Maintaining this homeostatic spatial organization comes at a metabolic cost. Cytoskeletal systems are major consumers of ATP:
- Actin polymerization and severing enzymes (e.g., cofilin, Arp2/3) use ATP to remodel filament networks.
- Myosin-II–driven contractility requires sustained ATP turnover to maintain cortical tension and mechanical stability.
- Microtubule dynamics are regulated by GTP hydrolysis and by ATP-powered motors guiding long-range transport.
This energy investment supports:
- Organelle positioning (e.g., mitochondria, Golgi, centrosomes)
- mRNA localization and translational hotspots
- Asymmetric signal distribution
- Cellular shape and motility readiness
The Cytoskeleton thus does not resist entropy by freezing the cell, but by maintaing an energetically charged, constantly renewing spatial order.
Nonequilibrium Stability: The Spatial Equivalent of ΔG
Just as ATP hydrolysis sustains chemical disequilibrium, cytoskeletal dynamics maintain spatial disequilibrium. The difference is that this form of order is geometrical:
- Distinct membrane domains
- Apical-basal polarity
- Organelle-specific localization zones
- Trafficking corridors and exclusion zones
These patterns are thermodynamic investments: the cell spends energy to keep itself asymmetrical, functionally compartmentalized, and capable of responding to directional cues.
In this phase, spatial coherence is stable because:
- ATP regeneration (e.g., mitochondrial function) meets the demands of filament turnover.
- Redox balance supports actin remodeling.
- Membrane tension and adhesion dynamics are tightly regulated through cytoskeletal feedback.
Summary: Spatial Order as a Rhythmic Investment
Dynamic homeostasis represents the baseline rhythm of cellular organization. Microtubules and actin filaments work together to:
- Resist entropic diffusion of structures
- Convert ATP into persistent spatial form
- Encode polarity, transport logic, and mechanical readiness
This phase is not about stasis, but about regulated flexibility—the capacity to move, adapt, and signal without descending into spatial noise. The cytoskeleton is a spatial entropy damper, transforming continuos energy flux into ordered intracellular architecture.
Disruption — Mismatch in Energy and Entropy Flux
Disruption marks the breakdown of intracellular spatial order—a collapse not just of cytoskeletal integrity, but of the thermodynamic coherence it sustains. In this phase, the energy cost of maintaining structured intracellular geometry exceeds the system’s regenerative capacity. Spatial gradients dissolve, transport becomes erratic, and structural polarity deteriorates.
This is not simply molecular damage but rather an entropic unraveling of spatial organization.
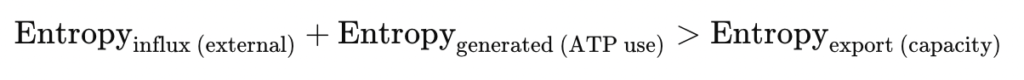
Common Triggers of Mitochondrial Disruption: Heat stress, Hypoxia, Acidosis, Oxidative or inflammatory stress
Consequences:
- AMP Accumulation: Signals net energy deficit. ATP hydrolysis outpaces regeneration, marking energetic collapse.
- Δψ / ΔpH Collapse: Proton gradient loss halts ATP production, especially in spatially distant or high-demand regions.
- ROS Accumulation: Oxidative damage to actin, tubulin, and motors fragments cytoskeletal networks and impairs transport.
- Calcium Overload: Mitochondrial Ca²⁺ buffering fails; cytosolic spikes activate calpain, degrade filaments, and destabilize membrane tension.
- Protein Unfolding: Heat and ROS overwhelm proteostasis, misfolding structural and contractile proteins before they can be refolded or cleared.
Consequences for the Spatial Architecture:
- Cytoskeletal Disassembly: Loss of filament integrity undermines shape, transport, and polarity.
- Motor Arrest: ATP shortage and oxidation stall kinesin, dynein, and myosin-driven movement.
- Organelle Drift: Vesicles and mitochondria become mislocalized; trafficking corridors collapse.
- Membrane Instability: Cortical breakdown leads to blebbing, leakage, or rupture.
- Entropy Spike: Coherent spatial geometry dissolves into stochastic distribution.
This phase ends with the passive collapse of spatial regulation. No containment has begun—only the clear signature of internal order giving way to thermodynamic entropy.
Reaction
Once spatial coherence is lost, the cell does not immediately repair itself — it defends. Reaction marks the first coordinated response to entropy overload. It is a short-term, high-cost containment phase, where the system urgently mobilizes internal reserves to halt further collapse and preserve minimal function.
Thermodynamic Context:
- ΔG (ATP hydrolysis) becomes less negative, reducing energy efficiency.
- ATP use increases, despite low regeneration
- ADP and Pi accumulate, contributing to metabolic strain.
- Entropy production is high, with misfolded proteins, ROS, and ion imbalance accumulating.
- Spatial polarity is disrupted, but the membrane and cytoplasm remain intact enough for response.
The cell enters a low-efficiency, high-output mode: a thermodynamic emergency state.
Mechanisms:
1. Metabolic Switching: AMPK-Orchestrated Emergency Reprogramming
Triggered by rising AMP:ATP ratios, AMPK becomes the central metabolic governor:
| Pathway | Response |
|---|---|
| Anabolism | Shut down (e.g., protein synthesis via mTOR inhibition) |
| Catabolism | Upregulated (e.g., glycolysis, fatty acid oxidation) |
| Autophagy | Initiated to recycle damaged components |
| Ion Transport | Energy is diverted to stabilize Na⁺/K⁺ and Ca²⁺ gradients |
Hardie (2011): AMPK allows the cell to reprioritize: spending less on growth, more on survival.
2. Cytoskeletal Stabilization Attempts:
With ATP regeneration still limited, the cell tries to reassert partial structure using minimal energy:
- Actin networks are remodeled into more compact, stress-bearing forms.
- Microtubule fragments are stabilized where possible (e.g., with MAPs or acetylation).
- Myosin-II activity is spatially redirected to maintain cortical integrity and limit blebbing.
3. Redox Defense and ROS Containment
To prevent oxidative chaos:
- Antioxidant systems (e.g., glutathione, catalase, SOD) are upregulated.
- Nrf2 pathways activate transcription of cytoprotective enzymes.
- ROS levels are not fully reversed—but their spread is checked to prevent irreversible damage.
ROS defense is energetically expensive, yet essential to contain molecular entropy.
4. Proteostasis Stress Buffering:
As misfolded proteins accumulate:
- Heat Shock Proteins (HSPs) refold damaged structural and motor proteins.
- The Unfolded Protein Response (UPR) slows translation and enhances ER-associated degradation.
- Chaperone systems attempt triage—rescuing salvageable proteins, tagging others for autophagic clearance.
Reaction does not restore proteome order but rather limits the damage from further misfolding.
5. Membrane and Mechanical Containment
To prevent rupture and ion leakage:
- Actin cortex is reinforced, often at the expense of intracellular remodeling.
- Calcium pumps and membrane repair machinery (e.g., ESCRT complexes) are mobilized.
- The cell reduces surface fluctuations and tries to preserve barrier integrity.
The goal of Reaction is to buy time: to prevent further disintegration while activating pathways that might enable adaptation. This phase is not sustainable: The system is still losing order, just more slowly.
Adaptation
Adaptation begins when containment has stabilized key variables—ATP levels, ROS, ion gradients—enough to allow a transition from emergency buffering to functional reprogramming. The goal is not to return to the pre-disruption state, but to establish a new sustainable order, tuned to the system’s altered conditions.
This phase restores both energy production and spatial architecture. The cytoskeleton is rebuilt, intracellular transport is reestablished, and ATP regeneration once again exceeds consumption.
1.Mitochondrial ATP Production
- ETC Complexes I–IV are repaired or replaced.
- Δψ and ΔpH are reestablished across the inner membrane.
- NAD⁺/NADH balance is restored to enable oxidative flux through the citric acid cycle.
- Mitophagy removes irreversibly damaged mitochondria; biogenesis (via PGC-1α, TFAM) replaces them.
Mitochondria become thermodynamically coherent again, generating ATP with high ΔG (~ –55 kJ/mol), supplying the energy needed for large-scale reordering.
In parallel:
- Glycolysis remains active to support adaptation under partial oxygenation or limited oxidative capacity.
- Regulated by AMPK, HIF-1α, and PFKFB3, glycolysis provides:
- Local ATP at sites distant from mitochondria
- Reducing equivalents (NADH) and intermediates for biosynthesis
While inefficient, glycolysis ensures localized energy availability where mitochondrial recovery is incomplete.
2. Cytoskeletal Reassembly and Spatial Repatterning
As ATP supply returns, the cell invests energy to rebuild spatial structure:
- Actin filaments re-polymerize, forming lamellipodia, stress fibers, and cortical scaffolds.
- Microtubules regrow from MTOCs (microtubule organizing centers), restoring intracellular highways.
- Motor proteins (e.g., kinesin, dynein, myosin) resume cargo delivery, membrane trafficking, and organelle positioning.
- Cell polarity is re-established through regulated anchoring of cytoskeletal domains.
Etienne-Manneville (2013): Cytoskeletal recovery is not just structural—it re-enables the cell’s informational and mechanical asymmetry.
4. Targeted Autophagy and Proteostasis Rebalancing
- Autophagy clears misfolded proteins, oxidized lipids, and organelles beyond repair.
- Chaperone systems (e.g., HSPs) return to basal activity after triage.
- Proteasomal degradation is reactivated to maintain protein quality control.
This process cleans up molecular noise, restoring informational clarity in the intracellular environment.
Thermodynamic Markers of Successful Adaptation:
- ATP regeneration ≥ ATP hydrolysis
- ΔG (ATP hydrolysis) becomes more negative → restored energy efficiency
- ROS levels normalize → antioxidant systems regain control
- Redox balance (NAD⁺/NADH) stabilizes
- Δψ and ΔpH recover → signaling, ion transport, and biosynthesis resume
- Entropy production slows, while entropy export mechanisms (e.g., heat, metabolic waste, autophagy) re-engage
The system exits the entropic deficit zone: energy input once again exceeds decay, allowing functional structure to be rebuilt.
Summary: Adaptation marks the thermodynamic reconstitution of the cytoskeleton following disruption. As mitochondrial ATP production recovers and glycolytic support stabilizes, the cell reinvests energy to reassemble and reorganize its internal scaffolding:
- Actin filaments are repolymerized to reestablish cortical integrity, lamellipodia, and contractile structures.
- Microtubules regrow from organizing centers, restoring directed intracellular transport and organelle positioning.
- Motor proteins are reactivated, enabling cargo delivery, polarity establishment, and vesicle flow.
- Mechanical asymmetry and spatial polarity are redefined, anchoring the cell in a new configuration of directional coherence.
- Structural entropy declines, not by reverting to a previous state, but by constructing a reoptimized cytoskeletal geometry suited to current metabolic and environmental constraints.
In essence, the cytoskeleton becomes the visible manifestation of the system’s adaptive logic: a reorganized spatial infrastructure built through precise energy allocation, signaling integration, and proteostatic clarity.
Refined Homeostasis
Refined Homeostasis is not a return to baseline — it is the emergence of a new spatial equilibrium. Following the energy crisis and structural collapse of earlier phases, and the restorative investment of Adaptation, the system stabilizes around a reorganized cytoskeletal configuration that reflects both stress history and functional priority.
1. Cytoskeletal Architecture Restored, But Repatterned
Unlike pre-disruption geometry, the restored cytoskeleton:
- Adopts new filament orientations aligned with updated mechanical and signaling demands.
- Redistributes organelles and vesicular traffic according to reweighted functional zones.
- Stabilizes anchoring points and polarity axes via microtubule-actin crosstalk and motor positioning.
The cytoskeleton becomes a memory structure of past disruption and adaptive prioritization.
2. Energy-Structure Coupling Optimized
- ATP hydrolysis is recoupled to spatial function: polymerization, transport, and tension generation are all powered precisely, with minimal waste.
- ΔG of ATP hydrolysis (~–55 kJ/mol) is sustained by high mitochondrial output and redox balance.
- Local ATP microdomains (e.g., at growth cones, synapses, immune synapses) match cytoskeletal demand with metabolic supply.
Energy is no longer spent reactively—it is invested strategically to maintain organized space.
3. Regulatory Resynchronization
- Oscillatory circuits (circadian, metabolic, cytoskeletal turnover) are re-entrained to the restored energetic landscape.
- Mechanotransduction feedback loops stabilize cytoskeletal dynamics via updated kinase/phosphatase balances (e.g., RhoA, Rac1, LIMK).
- Spatial signaling domains (e.g., leading edges, adhesion zones, immune synapses) operate with restored fidelity and directional precision.
4. Structural Memory and Plasticity
Refined Homeostasis encodes its adaptive trajectory:
- Epigenetic shifts (e.g., histone marks, chromatin organization) lock in stress-responsive expression of cytoskeletal genes.
- Organelle positioning is updated and maintained to match new transport needs.
- Cytoskeletal isoforms and post-translational modifications (e.g., tubulin acetylation, actin branching) reflect long-term recalibration.
The spatial organization is now more robust, more efficient, and primed for future perturbation.
Summary: A New Spatial Baseline:
Refined Homeostasis marks the end of the acute regulatory cycle and the birth of a new internal geometry: Spatial order has been re-established not as a restoration, but as a refinement and as an evolved attractor for coherent function.
The cytoskeleton is no longer reactive, but poised: tuned to support function under altered internal and external landscapes. Intracellular transport, polarity, and mechanical integrity are resynchronized with energy metabolism.
Conclusion
The cytoskeleton is not merely a structural scaffold — it is the spatial expression of a cell’s thermodynamic identity. Through dynamic coupling to energy flow and regulatory feedback, it enables living systems to sustain internal organization, directional transport, and mechanical resilience in a fundamentally disordered world.
This five-phase model reveals that spatial structure is not preserved by default. It must be continually re-established through energetic investment, entropy export, and adaptive modulation. As such, the cytoskeleton serves as a real-time thermodynamic barometer: reflecting the system’s capacity to maintain coherence, resist breakdown, and recalibrate under stress.
During Disruption, spatial order dissolves as the energetic foundation crumbles. Reaction buys time, containing entropy through high-cost stabilization. Adaptation leverages restored ATP production to rebuild and repattern internal architecture. Finally, Refined Homeostasis consolidates these changes into a new spatial equilibrium, tuned to both internal experience and external demand.
Crucially, this restored structure is not a passive return to form. It is a memory-bearing geometry: an evolved configuration encoding the lessons of past disruption. Through cytoskeletal reorganization, the cell redefines not only its shape, but its functional orientation in space — determining where energy is used, how force is transmitted, and where information flows.
In this light, the cytoskeleton becomes a dynamic boundary condition, constantly rewritten by thermodynamic context. It is where physics meets regulation, where entropy is made spatially meaningful, and where resilience is rendered visible in the language of structure.
References
Friston, K. (2010).
The free-energy principle: a unified brain theory?
Nature Reviews Neuroscience, 11(2), 127–138.
https://doi.org/10.1038/nrn2787
Hardie, D. G., Ross, F. A., & Hawley, S. A. (2012).
AMPK: a nutrient and energy sensor that maintains energy homeostasis.
Nature Reviews Molecular Cell Biology, 13(4), 251–262.
https://doi.org/10.1038/nrm3311
Vale, R. D. (2003).
The molecular motor toolbox for intracellular transport.
Cell, 112(4), 467–480.
https://doi.org/10.1016/S0092-8674(03)00111-9
Fletcher, D. A., & Mullins, R. D. (2010). Cell mechanics and the cytoskeleton. Nature, 463, 485–492.
https://doi.org/10.1038/nature08908
Etienne-Manneville, S. (2013). Microtubules in cell migration. Annual Review of Cell and Developmental Biology, 29, 471–499.
https://doi.org/10.1146/annurev-cellbio-101512-122308
Dominguez, R., & Holmes, K. C. (2011). Actin structure and function. Annual Review of Biophysics, 40, 169–186.
https://doi.org/10.1146/annurev-biophys-042910-155359
Mitchison, T., & Kirschner, M. (1984). Dynamic instability of microtubule growth. Nature, 312, 237–242.
https://doi.org/10.1038/312237a0
