Dynamic Homeostasis
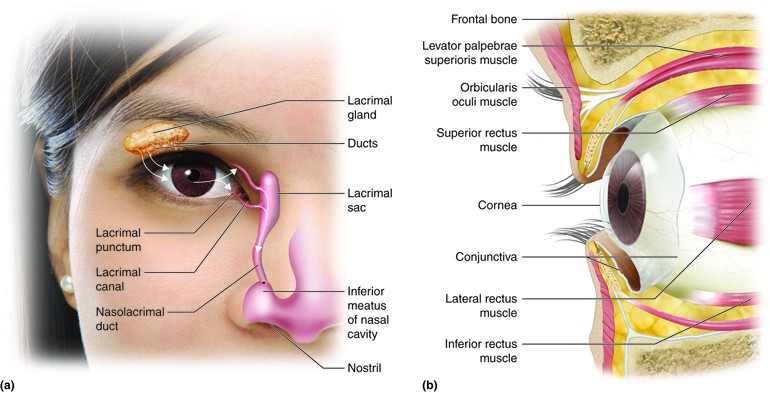
State: In dynamic homeostasis, the lacrimal functional unit — comprising the lacrimal gland, Meibomian glands, conjunctival goblet cells, corneal epithelium, eyelids, blink reflex arcs, and parasympathetic innervation — operates as a highly synchronized and adaptive feedback system.
The ocular surface remains moist, optically smooth, and immunologically protected due to a triphasic tear film (lipid, aqueous, mucin), maintained by coordinated neurosecretory, mechanical, and immune tolerance mechanisms. Tear drainage is likewise coupled to blink-induced pressure oscillations and ductal patency.
Key Regulatory Systems:
- Lacrimal Gland Acinar Cells (aqueous phase)
- Meibomian Glands (lipid phase)
- Conjunctival Goblet Cells (mucin phase)
- Blink Reflex Circuitry (facial + trigeminal integration)
- Parasympathetic and Sensory Innervation (CN VII, CN V1)
- Corneal Epithelium + Immune Tolerance (surface integrity, MUC1/MUC16)
Homeostatic Physiology and Feedback:
- Tear Film Assembly:
- Lipid layer (outermost): Secreted by Meibomian glands, reduces evaporation and enhances surface tension
- Aqueous layer (middle): Secreted by the main and accessory lacrimal glands, supplies nutrients, oxygen, antimicrobial peptides (e.g., lysozyme, lactoferrin), and fluid volume
- Mucin layer (innermost): Produced by goblet cells and membrane-bound mucins (e.g., MUC1, MUC16) — critical for tear film adhesion and surface hydration
- Immune-Epithelial Tolerance:
- Corneal and conjunctive epithelial cells: form tight junctions, express PRRs (Pattern Recognition Receptors), secrete TGF-ß
- Immature Dendritic Cells in the Conjunctival Stroma / CALT
- Tregs (Regulatory T-Cells): secrete IL-10, TGF-ß
- Plasma Cells in the lacrimal gland produce secretory IgA
- Cholinergic signals (PNS) may promote anti-inflammatory cytokine production
- Neurological Control:
- Basal secretion via tonic parasympathetic output: Nucl. salivatorius superior -> N. petrosus major -> Gangl. pterygopalatinum -> Azini
- Rapid secretion via sensory afferents: N. opthalmicus -> N. nasociliaris -> Nucl. salivatorius superior -> N. petrosus major -> Gangl. pterygopalatinum -> Azini
- Blink-Drainage Coupling:
- Blinking generates a coordinated pump mechanism by compressing and decompressing the canaliculi and lacrimal sac, creating pressure gradients:
- During eyelid closure, the orbicularis oculi (especially Horner’s muscle) contracts, compressing the canaliculi and lacrimal sac, which generates positive pressure that pushes tears downward into the nasolacrimal duct.
- Upon eyelid opening, the muscles relax, allowing the lacrimal sac to re-expand, which creates negative pressure (suction) that draws fresh tears in from the ocular surface via the puncta and canaliculi, refilling the lacrimal sac
- Eyelid-globe apposition ensures uniform spread and drainage with each blink (~15/min)
- Blinking generates a coordinated pump mechanism by compressing and decompressing the canaliculi and lacrimal sac, creating pressure gradients:
Symptoms:
- Moist, comfortable eyes without dryness or discharge
- Clear vision throughout the day
- No photophobia or foreign body sensation
- No excessive tearing or crusting
- Stable blink rate and eyelid tone
In this state, the system’s internal feedback loops are proactive, not reactive — preventing symptoms before they arise.
Therapeutic Goal: Preserve anticipatory and tonic tear secretion, maintain lipid barrier to prevent evaporation and maintain mucin-lipid-aqueous balance, support immune–epithelial tolerance without reactive inflammation, promote coordinated neural-epithelial interaction and drainage integrity
Prophylactic Interventions:
1. Lipid Barrier (Outermost Layer – Meibomian Glands) – primary defense against evaporation
- Regular blinking (~15x/min): mechanically spreads meibum across the ocular surface, reforming a uniform lipid sheet after each blink
- Omega-3 fatty acids: improve meibum quality and reduce gland obstruction or stagnation
- Androgen balance: promotes healthy lipid synthesis; androgen deficiency leads to Meibomian Gland Dysfunction (MGD)
- Environmental protection: humidifiers, moisture-retaining glasses, and reducing airflow prevent lipid destabilization due to physical stressors
- Visual ergonomics: screen breaks and full blinking restore lipid distribution, especially during prolonged visual tasks
2. Aqueous Barrier (Middle Layer – Lacrimal Glands)
- Circadian-aligned parasympathetic stimulation: basal tear flow follows daily rhythms and is suppressed by poor sleep or chronic stress.
- Vitamin A and estrogen: support lacrimal gland cellular metabolism and secretion.
- Anti-allergen strategies: reduce inflammation-driven aqueous suppression (e.g., antihistamines, allergen avoidance).
- Systemic hydration and avoiding anticholinergic medications also support volume
3. Mucin Barrier (Innermost Layer – Goblet Cells and Epithelial Glycocalyx): anchor to the hydrophobic corneal epithelium
- Avoiding preservatives: (especially benzalkonium chloride) in eye drops protects epithelial tight junctions and mucin secretion
- Vitamin A: maintains goblet cell density and mucin gene transcription
- Anti-inflammatory balance: chronic cytokine stress (e.g., IL-1β, TNF-α) destroys goblet cells.
- Allergen control: prevents conjunctival irritation and goblet cell loss.
Monitoring Tools:
| Tool | What It Reflects | Interpretation in Homeostasis |
|---|---|---|
| Schirmer I Test | Reflex and basal aqueous production | >10 mm = normal |
| Break-Up Time (BUT) | Stability of mucin-lipid interaction | >10 seconds = normal |
| Lissamine Green/Fluorescein | Epithelial health and tight junction integrity | No staining = intact barrier |
| Meibography | Meibomian gland structure | No dropout or truncation |
| Tear Osmolarity | Hydration equilibrium | 280–300 mOsm/kg = ideal |
| Conjunctival impression cytology | Goblet cell density and mucin expression | High density, MUC5AC presence |
Disruption
State: In this phase, the lacrimal functional unit begins to lose its capacity for fine-tuned, anticipatory regulation. Disruption refers to the early-stage breakdown of coordination across the secretory, mechanical, neurological, and surface epithelial systems — before overt clinical inflammation or immune pathology arises. The system shifts from a tonic and proactive equilibrium to a stressed and compensatory mode, characterized by subclinical instability.
Pathophysiology:
A. Temporal Disruption: Reflex Failure & Aberrant Inflammatory Timing
| Underlying Failure | Disease State | Pathophysiological Mechanism |
|---|---|---|
| Reflex arc latency (CN V1–VII) | Neurotrophic Keratopathy | Corneal hypoesthesia → loss of protective blink/tear reflex → persistent epithelial defects |
| Autonomic desynchronization | Nocturnal Lagophthalmos-related Dry Eye | Nighttime blink failure → evaporative stress and microtrauma during sleep |
| Tonic parasympathetic suppression | Stress-induced Dry Eye | Dysautonomia (e.g., in chronic stress or PTSD) reduces baseline aqueous flow rhythmically |
B. Spatial Disruption: Barrier Breakdown & Immune Exposure
| Spatial Instability | Disease State | Mechanism |
|---|---|---|
| Goblet cell degeneration | Allergic Conjunctivitis | Allergen-triggered cytokines (IL-4, IL-13) destroy goblet cells → mucin loss → barrier collapse |
| Mucin-aqueous Layer Decline | DED – Aqueous Deficient Type | Mucin scarcity → aqueous fails to hydrate → desiccation stress on epithelium |
| Lipid Layer Decline | Meibomian Gland Dysfunction (MGD) | Partial ductal obstruction → irregular lipid layer → focal evaporation zones |
| Tear stasis | Conjunctivochalasis | Redundant conjunctiva traps tears → stagnation → local inflammation & hyperosmolarity |
C. Energetic Dysregulation: Glandular Exhaustion & Immune Activation
| Energetic Failure | Disease State | Key Pathways |
|---|---|---|
| Lacrimal gland secretory degeneration | Sjögren’s Syndrome | Autoimmune infiltration → acinar apoptosis → loss of aqueous output |
| Meibomian gland metabolic suppression | Androgen-deficiency DED (Dry Eye Disease) (e.g., menopause) | Low androgen → decreased meibum synthesis enzymes → lipid starvation |
| Oxidative stress on goblet cells | Vitamin A deficiency keratoconjunctivitis | Antioxidant loss → epithelial metaplasia + mucin transcription failure |
| Sensory afferent silencing | Post-LASIK Dry Eye | Ablation of corneal nerves → reflex arc interruption → hyposecretion & neuroinflammation |
Symptoms:
- Meibomian Gland Dysfunction
- Intermittent blur, clears with blinking
- Evening visual “smear”
- Tear film awareness (“wet but dry”)
- Mild lid margin thickening, no gland dropout
- Evaporative Dry Eye (Non-MGD)
- Ocular fatigue late in day
- Reflex tearing in wind/AC
- Dry sensation without staining
- Slow tear film recovery post-blink
- Aqueous Deficiency – Subclinical Sjögren´s Syndrome:
- Morning stickiness or blur
- Reduced basal tearing (esp. AM)
- Dryness with normal Schirmer
- Mild photophobia, no epithelial damage
- Allergic / Irrative Stress:
- Mild foreign body sensation
- Reflex tearing, especially outdoors
- Thin mucous strands, no itch
- Fluctuating irritation, self-limited
- Conjunctivochalasis
- Intermittent tearing in downgaze
- Blurring that clears with blink
- Tear stagnation, irregular meniscus
- Normal secretion, poor clearance
- Neurotrophic Dry Eye
- Blinks feel incomplete or ineffective
- Ocular dryness without discomfort
- Fluctuating vision, reduced blink rate
- Decreased corneal sensitivity
- Hormonal Dry Eye (e.g, Menopause)
- Ocular heaviness in evening
- Subtle visual instability
- Reduced lipid quality, intact structure
- Tear film fatigue without inflammation
Therapeutic Goal: Preserve dynamic, proactive homeostasis by rebalancing secretory rhythms, restoring mechanical coordination, and supporting neuro-immune epithelial resilience — before pathological inflammation or immune activation occurs.
Clinical Application:
1.Temporal Regulation: Restore Physiological Timing
- Normalize blink frequency: Screen hygiene, behavioral prompts (~15/min)
- Support circadian secretion: Sleep quality, melatonin balance, light exposure
- Reduce stress-induced dysautonomia: Mind–body interventions (HRV training, stress management)
- Preserve sensory reflex latency: Protect corneal innervation (avoid preservatives, lens fatigue)
2.Spatial Reintegration: Stabilize Layered Interfaces
- Enhance tear film cohesion:
- Omega-3s → improve meibum fluidity
- Humidifiers → reduce evaporative microzones
- Protect mucin scaffold:
- Avoid preservatives (BAK-free drops)
- Early use of topical vitamin A or mucin secretagogues (e.g., rebamipide)
- Promote complete blinking:
- Blink training, visual ergonomics, warm compresses
3.Energetic Support: Sustain Secretory and Metabolic Output
- Support lacrimal and goblet cell metabolism:
- Adequate vitamin A, hydration, and hormonal balance (especially androgens)
- Enhance parasympathetic tone:
- Cholinergic-friendly practices (hydration, low anticholinergic load)
- Avoid suppressive medications (e.g., antihistamines, beta blockers)
- Reduce oxidative stress load:
- Early antioxidant support (e.g., topical lipoic acid, systemic omega-3s)
3. Reaction
State: In the Reaction Phase, the lacrimal system attempts to restore stability — but instead activates excessive or misdirected feedback, often through neuroimmune, epithelial, and glandular loops. This reaction is not passive. It represents an overcorrection, resulting in chronic symptoms, low-grade inflammation, and loss of immune tolerance.
Transitions:
| From Disruption | To Reaction |
|---|---|
| Reflex lag or fatigue | Incomplete blink syndrome |
| Lipid instability | Meibomian gland dropout |
| Mucin thinning | Goblet cell apoptosis |
| Tear stasis | Hyperosmolar inflammation |
| Neural desensitization | Neurotrophic keratitis |
| Stress-induced hyposecretion | Chronic aqueous deficiency |
| Surface microinjury | Cytokine cascade (IL-1β, MMP-9, TNF-α) |
Pathophysiology
- Neurogenic Inflammation:
- Sensory Signals trigger inflammatory amplification instead of coordination
- CN V1 overstimulation → Substance P, CGRP release
- Leads to vasodilation, leukocyte recruitment
- Example: Neuroinflammatory Dry Eye post-LASIK
- Sensory Signals trigger inflammatory amplification instead of coordination
- Immune-Epithelial Activation:
- Tolerance fails, epithelial stress becomes immunogenic
- Pattern Recognition Receptor (PRR) overactivation (TLRs, NLRs)
- Goblet cell loss exposes self-antigens → loss of mucosal tolerance
- MMP-9 cleaves tight junctions → worsens barrier damage
- Example: Allergic conjunctivitis
- Tolerance fails, epithelial stress becomes immunogenic
- Glandular Autoimmunity or Exhaustion:
- Excessive demand or immune infiltration leads to irreversible decline
- Chronic parasympathetic activation → acinar fatigue
- Autoimmune targeting (Sjögren’s) → apoptosis, fibrosis
- Meibocyte stress → keratinization and dropout
- Example: Primary Sjögren’s, advanced MGD
- Excessive demand or immune infiltration leads to irreversible decline
- Cytokine-Mediated Inflammation:
- IL-1β, TNF-α → goblet cell apoptosis
- IFN-γ → squamous metaplasia
- MUC gene repression (↓MUC5AC, ↑MUC1/MUC4 imbalance)
- Example: Chronic DED with conjunctival metaplasia
- Surface Instability Feedback Loop: Every blink aggravates the surface instead of replenishing it
- Inflammatory tears → increased osmolarity
- Epithelium sheds faster → more debris, more antigen
- Tear clearance slows → cytokine dwell time increases
- Example: DED with punctate epithelial keratopathy
Disease States:
- Dry Eye Disease (DED) – Inflammatory Stage:
- Symptoms: Persistent dryness, burning, tearing paradox
- Signs: Conjunctival staining, punctate epithelial erosions, low TBUT
- Biomarkers: ↑ MMP-9, ↑ tear osmolarity (>308 mOsm/kg), ↓ goblet cells
- Allergic Conjunctivits:
- Symptoms: Itch, tearing, mucus, foreign body sensation
- Pathology: Th2-mediated cytokines (IL-4, IL-5, IL-13) → eosinophils, goblet loss
- Complications: Shield ulcers, papillary hypertrophy if chronic
- Sjögren´s Syndrome / Autoimmune Lacrimal Dysfunction:
- Symptoms: Severe dryness, fatigue, arthralgia (systemic)
- Pathology: CD4+ T-cell infiltration, anti-SSA/SSB antibodies
- Consequences: Fibrosis, gland atrophy, loss of basal secretion
- Neurotrophic Keratopathy:
- Symptoms: Vision decline without discomfort
- Pathology: Loss of corneal nerves → epithelial breakdown
- Risk: Persistent epithelial defects, stromal melt, ulceration
- Meibomian Gland Degeneration / Obstructive MGD:
- Symptoms: Grittiness, dryness, worsens with blinking
- Pathology: Ductal keratinization, gland dropout (meibography)
- Secondary inflammation → lipid depletion loop
Therapeutic Goal:
- Interrupt inflammatory loops (e.g., corticosteroids, cyclosporine, lifitegrast)
- Restore immune tolerance (support Tregs, mucosal healing)
- Rebuild surface architecture (goblet cell recovery, amniotic therapies)
- Normalize nerve-epithelial interaction (serum tears, neuroprotective agents)
- Stabilize tear film triad (lipid, aqueous, mucin synergy restoration)
Clinical Application:
- Early Diagnostic Biomarker Use
- Tear Osmolarity Testing:
- 308 mOsm/kg or inter-eye difference >8 mOsm/kg: Indicates tear film instability and inflammation
- MMP-9 Detection (InflammaDry®):
- Positive = active surface inflammation: Guides decision for anti-inflammatory therapy
- Conjunctival Impression Cytology:
- Detects goblet cell loss, squamous metaplasia: Helps stage allergic and autoimmune ocular surface disease
- Meibography:
- Reveals gland dropout, ductal atrophy in MGD: Determines reversibility or need for regenerative approaches
- Tear Osmolarity Testing:
- Immunomodulatory Therapy
- Topical Cyclosporine A (e.g., Restasis®, Cequa®):
- Restores Treg dominance, reduces IL-2 signaling: Ideal for inflammatory dry eye and early Sjögren’s
- Lifitegrast (Xiidra®):
- Blocks LFA-1/ICAM-1 binding, reduces T-cell mediated ocular surface inflammation
- Topical Corticosteroids (Loteprednol, FML):
- For flare management or pre-treatment induction
- Short-term use to avoid IOP/glaucoma risk
- Topical Cyclosporine A (e.g., Restasis®, Cequa®):
- Gland and Surface Restabilization:
- Thermal Pulsation (e.g., LipiFlow®):
- Reopens obstructed meibomian glands
- Ideal in post-reaction MGD or with stagnant lipids
- Neurotrophic Support:
- Promote epithelial healing in neurotrophic keratopathy
- Contain EGF, vitamin A, fibronectin
- Amniotic Membrane (Prokera®):
- For goblet cell repopulation and anti-inflammatory signaling
- Used in advanced DED or persistent epithelial defects
- Thermal Pulsation (e.g., LipiFlow®):
- Targeted Allergen and Environment Management:
- Topical Antihistamines / Mast Cell Stabilizers:
- For allergic conjunctivitis (olopatadine, ketotifen): Preserve goblet cells and mucin expression
- Environmental Controls:
- Humidification, moisture goggles, airflow redirection
- Reduce evaporative and hyperosmolar stress
- Topical Antihistamines / Mast Cell Stabilizers:
- Restoration of Tear Film Composition:
- Lipid-based artificial tears
- Restore outermost layer, stabilize film
- Useful in post-reaction lipid collapse (MGD)
- Mucin secretagogues (e.g., rebamipide)
- Enhance MUC5AC expression
- Hydrophilic gel or HA-based tears:
- Mimic aqueous function, protect epithelial surface
- Lipid-based artificial tears
- Monitoring & Staging:
- Reaction-phase interventions are time-sensitive
- Delayed care risks irreversible remodeling
- Use of structured dry eye questionnaires (OSDI, DEQ-5)
- Combination of subjective symptoms + objective inflammation markers
- Reassessments after 4–8 weeks of immunomodulatory treatment to confirm response
Adaptation
State: The Adaptation Phase represents a stabilized but altered state of the ocular surface. The system no longer tries to return to its original baseline — instead, it adopts structural, cellular, and neuroimmune changes that allow continued function in a degraded or inflamed environment.
This phase reflects functional compromise, not active inflammation, and is often clinically silent but irreversible. This phase is functionally stabilizing, but pathologically progressive.
Key Systems:
| Mechanism | Purpose | Cost |
|---|---|---|
| Tissue remodeling | Reduce immune visibility, limit damage | Loss of goblet cells, fibrosis |
| Neuroadaptation | Dampen symptom severity | Sensory desensitization, neurotrophic risk |
| Reflex suppression | Avoid inflammatory overactivation | Hypolacrimation, impaired healing |
| Secretory reprogramming | Stabilize tear film with fewer resources | Mucin shifts, lipid depletion |
Pathophysiology:
- Epithelial Remodeling:
- Goblet cell metaplasia or depletion
- MUC gene rebalancing (↓MUC5AC, ↑membrane-bound MUC1/MUC16)
- Squamous metaplasia of conjunctiva
- Clinical sign: Loss of conjunctival transparency, mild staining with Lissamine green
- Glandular Exhaustion and Atrophy
- Lacrimal gland fibrosis in Sjögren’s or radiation exposure
- Meibomian gland dropout in late-stage MGD
- Ductal stenosis or acinar collapse
- Meibography: Gland truncation, loss of architecture
- Reduced Neurological Feedback
- Reduced corneal sensitivity (neurotrophic state)
- Reflex arc desensitization (↓CN V1 → CN VII coupling)
- Loss of blink reflex urgency
- Clinical paradox: Patient reports “better” despite worsening surface disease
- Chronic Tear Film Instability
- Persistent low TBUT
- Osmolarity fluctuates but remains high-normal
- Incomplete blinking patterns normalize
- Patient adaptation: Increased tolerance for blur or dryness
Symptoms:
- Blunted Dryness Sensation due to corneal desensitization
- Chronic Mild Blur due to Tear film instability
- Ocular Fatigue
- Paradoxical Tearing due to poor drainage or reflex hypersecretion
- Delayed Recovery after minor stress
- Mucous Debris / Stickiness due to goblet cell dysfunction, mucin imbalance
Therapeutic Goal: Interrupt maladaptive stability by reversing cellular memory, re-sensitizing regulatory axes, and restoring anticipatory signaling.
Clinical Application:
- Regeneration: Residual Gland Function
- Omega-3 fatty acids (2–3 g/day) — meibum quality
- Low-dose cyclosporine A (Restasis®, Cequa®) — anti-inflammatory maintenance
- Reinforce Ocular Surface Integrity
- Autologous serum tears (20–100%) — epithelial trophic support
- Topical vitamin A (retinol palmitate) — goblet cell health
- Stabilize Tear Film
- Lipid-based artificial tears (e.g., Systane Balance®, Retaine®) — restore lipid layer
- Hyaluronic acid gels or cross-linked HA — hydration, protection
- Control Epiphora and Improve Drainage
- Punctal occlusion (plugs or cautery) — if non-inflammatory aqueous deficiency
- Treat conjunctivochalasis surgically — in mechanical tear clearance issues
- Restore Blink Function and Neurofeedback
- Blink training, screen hygiene — for partial blink patterns
- Neuropathic support: omega-3s, serum tears, consider Cenegermin† in neurotrophic cases
- Protect from Environment
- Moisture goggles, humidifiers, wraparound glasses — reduce evaporation
Refined Pathological Homeostasis
State: Following repeated or unresolved cycles of disruption and maladaptive adaptation, the CNS enters a dysregulated but stable condition
Key Events:
| Domain | Description |
|---|---|
| Structure | Permanent tissue changes (e.g., gland dropout, squamous metaplasia) |
| Function | Reduced secretory capacity, altered blink patterns |
| Immunity | Low-grade tolerance, absence of overt inflammation |
| Symptoms | Stable, plateaued symptoms or complete desensitization |
| Response to Therapy | Minimal improvement; focus is on maintenance and preservation |
Pathophysiology:
- Tear Film: Thinner, hyperosmolar, slow turnover
- Glands: Irreversible loss or fibrosis in lacrimal/meibomian tissue
- Conjunctiva: Keratinized or metaplastic, goblet cell loss
- Neurosensory: Dampened reflex arc, low corneal sensitivity
- Mucins: ↓MUC5AC, ↑MUC1/MUC16 (membrane-bound dominance)
Symptoms:
- Minimal or absent symptoms despite poor clinical findings
- Chronic visual haze or “soft focus” baseline
- Eye fatigue during high visual demand
- Increased tolerance to dryness — reflects neurosensory desensitization
- Slow recovery after stress or injury
Therapeutic Goal: At this stage, therapy is no longer corrective — it is conservational. The goal is to prevent regression into inflammation, support remaining function, and enhance quality of life.
Clinical Application:
- Sustain surface hydration
- HA-based artificial tears, gel drops
- Scleral lenses for stable hydration and refractive support
- Preserve epithelial health
- Autologous serum tears (maintenance)
- Topical vitamin A (retinoid support)
- Maintain ocular surface immunity
- Low-dose cyclosporine A or lifitegrast as needed
- Omega-3 fatty acids for anti-inflammatory balance
- Protect from environmental triggers
- Moisture chamber eyewear, humidifiers
- Behavioral: screen breaks, blink optimization
Biomarkers
| Tool / Test | What It Measures | Interpretation | Phase Sensitivity |
|---|---|---|---|
| Tear Break-Up Time (TBUT) | Tear film stability | <10 sec = unstable film | Disruption → Reaction |
| Schirmer I Test | Aqueous tear production | <5 mm in 5 min = deficiency | Reaction → Adaptation |
| Tear Osmolarity | Tear concentration | >308 mOsm/kg or >8 mOsm/kg inter-eye difference = abnormal | Disruption → Refined Homeostasis |
| MMP-9 (InflammaDry®) | Surface inflammation marker | Positive = active inflammation (IL-1β, TNF-α activity) | Reaction |
| Meibography | Meibomian gland structure | Dropout, truncation, tortuosity = MGD | Disruption → Adaptation |
| Lissamine Green / Fluorescein Staining | Epithelial damage, barrier loss | Positive = conjunctival or corneal epithelial compromise | Reaction → Adaptation |
| Conjunctival Impression Cytology | Goblet cell density, squamous metaplasia | ↓MUC5AC, keratinization = mucin dysfunction | Adaptation → Refined Homeostasis |
| Corneal Esthesiometry | Corneal sensory nerve function | ↓ Sensitivity = neurotrophic state | Adaptation → Refined Homeostasis |
| Non-Invasive Tear Meniscus Height (NITMH) | Tear reservoir volume | <0.2 mm = aqueous insufficiency | Disruption → Reaction |
| Blink Analysis (Video or Sensor-Based) | Blink completeness and rate | Infrequent or partial blinking = lipid instability | Disruption |
Conclusion
The lacrimal functional unit maintains ocular surface integrity through a highly synchronized network of secretory, mechanical, immune, and neurological systems. When this dynamic homeostasis is disrupted, the system enters a cascade of progressive phases: Disruption, Reaction, Adaptation, and ultimately, a Refined New Homeostasis.
Each phase reflects a shift from proactive regulation to reactive compensation, characterized by distinct pathophysiological mechanisms, biomarkers, and clinical presentations. Crucially, the system does not collapse all at once — it attempts to adapt through remodeling, suppression, or reprogramming, often at the cost of functionality and resilience.
Understanding these phases may allow for earlier interventions, target the right mechanisms, and personalize treatment; not only to suppress inflammation but to preserve anticipatory feedback, support residual function, and prevent irreversible degeneration.
Lacrimal disorders exemplify systems biology: disease emerges not from collapse alone, but from maladaptive survival strategies embedded in reactive feedback. Recognizing and treating these states before permanent remodeling occurs offers the best chance to preserve visual comfort, surface stability, and ocular health.
Abbreviations List
Anatomy & Physiology
LFU – Lacrimal Functional Unit
MGD – Meibomian Gland Dysfunction
CALT – Conjunctiva-Associated Lymphoid Tissue
CN – Cranial Nerve
CN V1 – Ophthalmic branch of the Trigeminal Nerve
CN VII – Facial Nerve
Azini – Acinar cells (of lacrimal or Meibomian glands)
Tear Film Components
MUC1/MUC4/MUC5AC/MUC16 – Mucin genes/proteins (membrane-bound and secreted forms)
PRR – Pattern Recognition Receptor
TGF-β – Transforming Growth Factor Beta
IL-1β, IL-4, IL-5, IL-10, IL-13 – Interleukins (key cytokines in immune signaling)
IFN-γ – Interferon gamma
MMP-9 – Matrix Metalloproteinase 9
Diagnostics & Tests
TBUT – Tear Break-Up Time
OSDI – Ocular Surface Disease Index
DEQ-5 – Dry Eye Questionnaire-5
NITMH – Non-Invasive Tear Meniscus Height
MMP-9 – Matrix Metalloproteinase-9 (inflammatory biomarker test: InflammaDry®)
Therapies & Drugs
HA – Hyaluronic Acid
BAK – Benzalkonium Chloride (preservative)
EGF – Epidermal Growth Factor
NGF – Nerve Growth Factor
NGF (rhNGF) – Recombinant Human Nerve Growth Factor (e.g., Cenegermin/Oxervate®)
Immunology
Tregs – Regulatory T Cells
Th1 / Th2 / Th17 – T Helper Cell Subtypes
SSA/SSB – Autoantibodies associated with Sjögren’s Syndrome
References
Bron, A. J., de Paiva, C. S., Chauhan, S. K., Bonini, S., Gabison, E. E., Jain, S., … & Sullivan, D. A. (2017).
TFOS DEWS II Pathophysiology Report. The Ocular Surface, 15(3), 438–510.
https://doi.org/10.1016/j.jtos.2017.05.011
Bron, A. J., de Paiva, C. S., Chauhan, S. K., et al. (2017). TFOS DEWS II Pathophysiology Report. Ocular Surface, 15(3), 438–510.
https://doi.org/10.1016/j.jtos.2017.05.011
Pflugfelder, S. C., & de Paiva, C. S. (2017). The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ocular Surface, 15(2), 331–338.
https://doi.org/10.1016/j.jtos.2017.03.003
Knop, E., Knop, N., Millar, T., Obata, H., & Sullivan, D. A. (2011). The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Anatomy, Physiology, and Pathophysiology of the Meibomian Gland. Investigative Ophthalmology & Visual Science, 52(4), 1938–1978.
https://doi.org/10.1167/iovs.10-6997c
Jones, L., Downie, L. E., Korb, D., et al. (2017). TFOS DEWS II Management and Therapy Report. Ocular Surface, 15(3), 575–628.
https://doi.org/10.1016/j.jtos.2017.05.006
Ji, Y. W., Lee, H., Choi, W., et al. (2018). Tear MMP-9 Levels and Ocular Surface Parameters in Primary Sjögren’s Syndrome. Yonsei Medical Journal, 59(1), 116–122.
https://doi.org/10.3349/ymj.2018.59.1.116
McMonnies, C. W. (2015). The Potential Role of Neurogenic Inflammation in Dry Eye Syndromes. Ophthalmic & Physiological Optics, 35(6), 545–554.
https://doi.org/10.1111/opo.12238
Ríos, J. D., Horikawa, Y., Chen, L. L., et al. (2005). Immune Regulation of Ocular Surface Homeostasis. Progress in Retinal and Eye Research, 24(1), 1–27.
https://doi.org/10.1016/j.preteyeres.2004.06.002
Symes, R., & Benjamin, L. (2020). Conjunctivochalasis: An Updated Review. Clinical & Experimental Optometry, 103(6), 835–841.
https://doi.org/10.1111/cxo.13070
