Imagine your immune system as a highly trained army, always on the lookout for invaders. But here’s the twist: what if your immune system could be trained better—with the right strategy, the right commanders, and the right battle plan? What if cancer didn’t stand a chance against an army that knew exactly how to find and destroy it?
The key to this vision lies in the immune system’s generals: dendritic cells. These unsung heroes are responsible for scouting out threats, gathering critical intelligence, and directing the immune system’s T-cells to fight. But what if we could make these generals even smarter, giving them the power to rally the immune system against cancer with unprecedented precision? This is the promise of dendritic-based immunotherapy—a revolutionary approach that has the potential to transform how we fight cancer.
Are we on the brink of an immunotherapy revolution? Can we train the body’s generals to recognize and eradicate cancer more efficiently than ever before? Let’s dive into the exciting world of dendritic cell-based therapies and explore how they could become the key to a smarter, more targeted cancer treatment strategy.
Dendritic Cells: What Makes These Immune “Generals” So Crucial?
Dendritic cells are the brainy generals of your immune system. They act as intelligence officers, capturing fragments of invaders (called antigens) and presenting them to the immune system’s frontline warriors—T-cells. When properly trained, these T-cells become equipped to fight off threats, especially cancer cells.
How Dendritic Cells Drive Immune Response
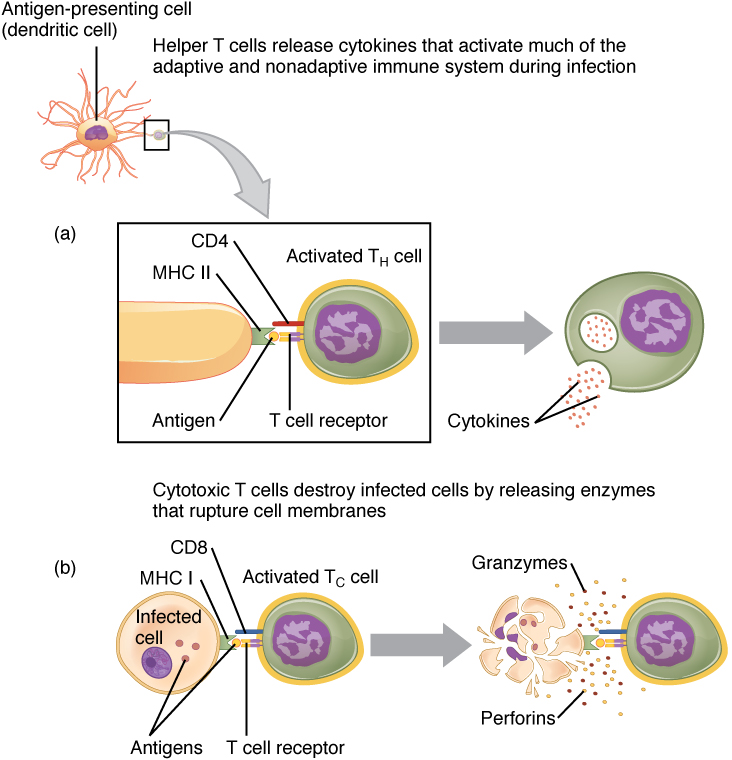
Dendritic cells activate T cells through a process called antigen presentation. Here’s how it works:
- Capture Antigens: Dendritic cells patrol tissues and capture foreign antigens (like pathogens or tumor cells) by engulfing them
- Migration to Lymph Nodes: After capturing the antigen, dendritic cells mature and migrate to the nearest lymph node
Antigen Presentation: In the lymph node, dendritic cells present fragments of the antigen on their surface using molecules called MHC (Major Histocompatibility Complex)
There are two main types of MHC:
a) MHC Class II: Presents to CD4+ helper T cells
b) MHC Class I: Presents to CD8+ cytotoxic T cells
- T Cell Activation: When a T cell’s receptor (TCR) recognizes the presented antigen on the MHC molecule, the T cell becomes activated
Response Initiation: Once activated, the T cell proliferates and differentiates into specific types, such as cytotoxic T cells (to kill infected cells) or helper T cells (to coordinate the immune response)
How Dendritic-Based Immunotherapy Works: Can We Outsmart Cancer with Smarter Immunity?
Dendritic-based immunotherapy takes advantage of the natural intelligence of these generals. Doctors collect a patient’s dendritic cells and train them in a lab to recognize cancer-specific markers. These trained dendritic cells are then reintroduced into the patient’s body. Once back in the system, they activate the immune response, guiding T-cells to directly attack and destroy the cancer cells.
Imagine it as sending highly trained generals back into battle, but now with detailed intelligence about the enemy. It’s not just about random attacks—it’s a targeted, smart assault.
Expanding the Landscape: DC-Based Vaccines vs. Other Cancer Vaccines
While dendritic cell-based vaccines offer a promising and personalized approach, they are not the only type of cancer vaccines currently in development. Other immunotherapy strategies, such as protein-based vaccines, viral vector vaccines, and mRNA vaccines, are also being explored in the fight against cancer.
But why might dendritic cell-based vaccines stand out from these other approaches?
Unlike traditional cancer vaccines that focus solely on delivering tumor antigens to the immune system, dendritic cell vaccines take a more targeted, multi-step approach. By using a patient’s own dendritic cells, which are trained in the lab to recognize specific cancer markers, these vaccines aim to re-educate the immune system to more effectively seek out and destroy cancer cells.
Moreover, because dendritic cells are the body’s most powerful antigen-presenting cells, they can help bridge the gap between the innate (body’s first line of defense) and adaptive (body´s second line of defense) immune responses—enhancing the overall immune attack against the tumor.
A significant advantage of dendritic cell vaccines over protein-based or viral vector vaccines is their ability to personalize treatment. By using tumor-specific antigens or neoantigens directly from the patient’s cancer, dendritic cell vaccines aim to generate a highly tailored immune response, potentially leading to better outcomes with fewer side effects.
As exciting as these other cancer vaccine technologies are, dendritic cell-based vaccines continue to emerge as a key player in the battle against cancer, especially when used in combination with other therapies such as immune checkpoint inhibitors or CAR-T cell therapies.
Ongoing research and clinical trials may soon make them a more widespread and personalized option for patients.
Dendritic-Based Immunotherapy vs. CAR-T Cell Therapy: Which Strategy Holds the Most Promise for the Future?
Now, let’s take a moment to compare this with CAR-T cell therapy, another breakthrough in cancer treatment. If dendritic-based immunotherapy is about training skilled generals to coordinate a strategic battle, CAR-T therapy is more like creating elite commandos.
In CAR-T therapy, your T-cells are extracted, genetically modified to target cancer markers, and then reinfused. These modified T-cells are like a team of commandos, trained and prepared to go after one specific target.
While CAR-T therapy is showing great promise for blood cancers, it faces challenges with solid tumors—like those found in organs such as the lungs or liver. Solid tumors are more difficult to treat because of their physical structure and location.
Dendritic-based immunotherapy, on the other hand, offers more flexibility and adaptability, making it a compelling approach for a wider variety of cancers—including those that have traditionally been difficult to treat, like solid tumors.
The Role of Lipopolysaccharide (LPS): Can We Supercharge Your Immune Generals?
Here’s where the research gets even more fascinating. A recent study has shown that lipopolysaccharide (LPS), a substance that triggers a powerful immune response, can significantly boost the effectiveness of dendritic cells. When dendritic cells are exposed to LPS, they mature faster and become potent—in other words, stronger and more effective at stimulating the immune system.
Maturation refers to the process where dendritic cells develop into fully functional, active immune fighters. It’s like training them from recruits into expert generals. These potent cells can then guide your immune system’s T-cells to launch a much stronger attack against cancer cells. This could make all the difference in how well your immune system fights back.
Personalized DC Vaccines: What if Your Immune System Had a Custom Battle Plan?
But it doesn’t stop there. What if we could tailor this strategy to your specific cancer? That’s where personalized dendritic cell vaccines come in. Doctors can create a vaccine specifically for you by using pieces of your own tumor, called tumor lysates, or by identifying unique changes in your tumor’s DNA, known as neoantigens. These pieces are used to “teach” your dendritic cells to recognize and attack your cancer with incredible precision.
- Tumor lysates are broken-down pieces of cancer cells.
- Neoantigens are unique proteins found on cancer cells that your immune system can target.
This personalized approach ensures that your immune system has the exact intelligence it needs to target your cancer more accurately, helping to prevent collateral damage to healthy tissues.
💡For more information on the power of dendritic cell-based vaccines in immunotherapy, click here.
Challenges: How Can We Overcome the Blood-Brain Barrier to Treat Brain Tumors?
While dendritic-based immunotherapy shows tremendous promise, it’s not without its challenges—particularly when it comes to treating brain tumors like glioblastoma (GBM). Glioblastomas are a type of brain tumor that are particularly aggressive and difficult to treat. These tumors are often resistant to traditional therapies and can spread quickly within the brain.
Brain tumors are tricky because they are protected by the blood-brain barrier (BBB), which is a natural defense mechanism that prevents harmful substances, including immune cells, from entering the brain. This protection, while important for safeguarding the brain, also makes it difficult to deliver immune therapies to these tumors.
Moreover, glioblastomas create a highly immunosuppressive environment—meaning they actively suppress the body’s immune response, making it harder for the immune system to attack the tumor. But could this be overcome? What if we could get our dendritic cells into the brain, bypassing the BBB and empowering the immune system to break through that barrier?
Researchers are already exploring solutions. Direct injection of dendritic cells into the tumor or cerebrospinal fluid (CSF), the fluid that surrounds the brain and spinal cord, could bypass the BBB. Using nanoparticles to carry tumor antigens, or even temporarily opening the blood-brain barrier with focused ultrasound, could all improve dendritic cell migration to the tumor.
Monocytes: What Role Do These Immune Cells Play in Cancer Vaccines?
Recent research has uncovered another critical piece of the puzzle. Monocytes—the precursor cells that eventually become dendritic cells—are a key starting material for creating cancer vaccines.
But here’s the catch: the conditions under which monocytes are isolated can greatly impact the yield of dendritic cells, their maturation, and their ability to effectively stimulate the immune system.
Monocyte isolation is the process of extracting these monocytes from a patient’s blood. Once isolated, these monocytes can be transformed into dendritic cells in the lab, where they can be trained to target cancer cells.
In a study focused on pediatric patients with high-risk sarcomas and neuroblastoma, researchers found that chemotherapy and targeted therapies could impair the quality of the dendritic cells derived from the patient’s monocytes. This suggests that while chemotherapy may enhance the visibility of tumor antigens, the timing of these treatments is crucial.
Combining chemotherapy with dendritic cell therapy isn’t just about targeting the cancer; it’s also about making sure the monocytes used to create the vaccine are still effective in producing strong, immunostimulatory dendritic cells.
- Sarcoma is a type of cancer that starts in the connective or supportive tissues, such as muscles, bones, or cartilage.
- Neuroblastoma is a cancer that most commonly affects children and begins in the nerve tissue, often in the adrenal glands.
In essence, this research emphasizes the importance of carefully considering the drug combinations and dosing schedules used alongside dendritic cell therapy. The right approach could unlock even greater potential in immunotherapy, improving both the quantity and quality of dendritic cells that fight cancer.
Conclusion: Could Dendritic Cell Immunotherapy Be the Key to Smarter, More Personalized Cancer Treatments?
Dendritic-based immunotherapy represents a groundbreaking shift in cancer treatment. Rather than relying solely on the brute force of chemotherapy or radiation, this strategy taps into the intelligence of your immune system, empowering it to recognize and destroy cancer with greater precision. And as science advances, we can expect these therapies to become more personalized, more effective, and more accessible.
But it’s not just about what we can do today—it’s about what’s coming next. The integration of personalized medicine, cutting-edge research, and AI-driven insights will likely refine dendritic cell therapies, making them even more effective. Could we one day personalize these vaccines so accurately that each patient receives a treatment that’s tailored not just to their tumor, but to their unique immune response? What if this approach could eventually revolutionize the way we treat all cancers, giving us a smarter, more effective weapon in the fight?
The future is filled with possibilities. And as we continue to unlock the potential of dendritic cell-based therapies, the fight against cancer is about to get a whole lot smarter.