Dynamic Homeostasis – Persistence from Thermodynamic Equilibrium
Living systems persist in a state of energetic tension: they are thermodynamically open, continuously exchanging matter and energy with their environment, yet they maintain local order in the face of global entropy. Unlike closed systems that drift passively toward equilibrium, cells actively resist disorder by managing the flow of free energy and entropy across structured boundaries—from the lipid membrane of a single cell to the epithelium of tissues and the skin of organisms.
The first internal boundary where energy is converted into structure is the mitochondrion. Through oxidative phosphorylation, mitochondria transform high-energy electrons into ATP and heat, generating electrochemical gradients (Δψ, ΔpH) that sustain intracellular coherence.
At the core of this regulation lies a continuous balancing act:
- Importing free energy substrates (e.g., glucose, oxygen),
- Exporting entropy in the form of heat, waste, and molecular disorder,
- Maintaining the structural integrity of subsystems required for functional order.
Contrary to classical homeostatic models that assume a return to static setpoints, biological regulation is inherently non-equilibrium and phase-dependent. It unfolds as a dynamic trajectory across five interrelated phases:
- Dynamic Homeostasis – Low-cost regulation under steady-state conditions
- Disruption – Mismatch between entropy influx and dissipation capacity
- Reaction – Acute, energy-intensive containment of rising disorder
- Adaptation – Structural reorganization and functional reprogramming
- Refined Homeostasis – Emergence of a reorganized regulatory baseline
These phases reflect distinct thermodynamic states—each shaped by the cell’s ability to manage internal entropy.
In alignment with the Second Law of Thermodynamics, biological systems do not violate the principle of increasing entropy. Instead, they offset internal entropy production by continuously exporting disorder to the environment, enabling the maintenance of localized low-entropy states.
Biological systems export entropy through:
- Heat dissipation from exergonic reactions and ion pumping
- Excretion of low-energy byproducts (CO₂, urea, ammonia)
- Structural cycling, including protein turnover, cytoskeletal remodeling, membrane renewal
- Electrochemical work, such as maintaining proton gradients (ΔpH), membrane potential (Δψ), and ion transport (Na⁺/K⁺-ATPase)
These processes constitute the energetic infrastructure of homeostasis, allowing cells to function despite the constant thermodynamic drive toward disorder.
ATP—the universal energy currency—mediates this balance.
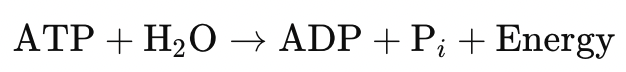
Under physiological conditions, ATP hydrolysis is strongly exergonic (ΔG ≈ –50 to –60 kJ/mol). Synthesized within mitochondria, it provides the free energy required for:
- Ion transport
- Macromolecular assembly
- Cytoskeletal and contractile work
- Signal transduction
Yet ATP hydrolysis itself increases entropy by:
- Producing disordered byproducts (ADP + Pi)
- Generating thermal energy
- Driving secondary reactions, such as ROS production
The sustainability of this energy use depends on mitochondrial integrity. Efficient ATP regeneration requires intact proton gradients, sufficient oxygen, and proper redox balance (NAD⁺/NADH). Mitochondria thereby anchor the cell’s capacity to resist entropy—not only by producing ATP, but by regulating the gradients and feedback loops that enable long-term systemic coherence.
Disruption — Mismatch in Energy and Entropy Flux
Disruption begins at the mitochondrial level—the first internal boundary where energy is structured into biological order. As the primary site of ATP regeneration and redox regulation, mitochondria serve as the thermodynamic backbone of cellular coherence. When their function is impaired, a cascade of dysregulation unfolds, not as isolated molecular failures, but as a systemic collapse of entropy management.
Despite ongoing energy demand, mitochondrial dysfunction halts the regeneration of ATP. Proton gradients (Δψ, ΔpH) across the inner mitochondrial membrane dissipate, oxidative phosphorylation stalls, and electron leakage from complexes I and III amplifies reactive oxygen species (ROS) production. These ROS—byproducts of disrupted electron flow—damage lipids, proteins, and mitochondrial DNA, further accelerating internal disorganization.
ATP hydrolysis continues during this phase, as enzymatic systems remain active. However, with regeneration impaired, the cell enters a net-entropy state: energy is consumed without structural restitution. This uncoupling between ATP use and ATP renewal marks a shift from regulated function to entropic oversaturation.
Common Triggers of Mitochondrial Disruption:
- Heat stress – destabilizes protein conformation and mitochondrial membrane integrity
- Hypoxia – deprives mitochondria of oxygen, the terminal electron acceptor
- Acidosis – impairs enzyme activity and alters membrane potential
- Oxidative or inflammatory stress – overwhelms antioxidant capacity, exacerbating ROS accumulation
Thermodynamic Events:
- Collapse of Δψ and ΔpH – proton gradients dissipate, halting ATP synthesis
- NAD⁺/NADH imbalance – stalls the citric acid cycle and complex I function
- Electron leak – increases ROS generation, particularly under low O₂ or high membrane tension
- Protein unfolding – activates the unfolded protein response (UPR) and heat shock proteins
- Calcium dysregulation – mitochondrial permeability transition pores may open, amplifying failure
- Ion gradient loss – Na⁺, K⁺, and Ca²⁺ gradients collapse, impairing membrane excitability and signaling
System Consequences:
Disruption is not merely biochemical degradation—it is a multidimensional breakdown of regulatory coherence:
- Temporal: Rhythmic control systems—circadian oscillators, redox cycles, metabolic clocks—desynchronize.
- Spatial: Intracellular and intercellular compartments operate out of sync; signaling becomes noisy and uncoordinated.
- Energetic: ATP hydrolysis proceeds even as its yield (ΔG) declines, due to high ADP/Pi levels and weak mitochondrial drive.
Here, regulation does not fail actively—it fails passively. The system exceeds its entropy-buffering capacity, spending energy to no productive end. Structure is lost faster than it can be repaired.
- Rising AMP – indicates energy deficit, triggering AMPK but not reversing damage
- Less negative ΔG (ATP hydrolysis) – reflects reduced energy output per molecule
- Weakened electrochemical gradients – compromises ion homeostasis and transport
- Elevated internal entropy (ΔS) – detectable as increased molecular disorder, noise, and instability
AMP serves here not as a signal of control, but as a symptom of exhaustion. While it activates compensatory pathways (e.g., AMPK), it cannot on its own restore mitochondrial gradients or rebuild structure. The cell enters a state of thermodynamic fragility, where coherence decays despite ongoing energy use—a prelude to either collapse or reorganization.
Reaction — Acute Compensation and Containment
Following mitochondrial disruption, the system enters the Reaction phase—a transient, high-cost state aimed at preventing collapse. Reaction is not recovery; it is containment. It reflects the cell’s acute awareness of internal entropy accumulation and the urgent deployment of short-term compensatory responses to stabilize core energetic and structural functions.
Reaction begins when critical thermodynamic thresholds are crossed. These are not predictive regulators but reactive inflection points—signals that entropy has exceeded buffer capacity and the system is operating beyond sustainable control.
Thermodynamic Triggers
- AMP accumulation → Activates AMPK, initiating a switch from anabolism to catabolism
- ROS surge → Induces expression of antioxidant enzymes (glutathione, catalase, superoxide dismutase)
- Calcium influx → Disrupts cytoskeletal stability, triggers membrane repair and mitochondrial permeability responses
- Protein unfolding → Engages heat shock proteins (HSPs) and the unfolded protein response (UPR)
- Collapse of Δψ / ΔpH → Further suppresses mitochondrial ATP output, forcing reliance on glycolytic backup
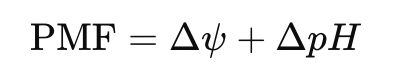
These triggers represent threshold responses, not feedback loops. They initiate rapid, localized containment to prevent further loss of coherence but do not restore systemic balance.
Containment Mechanisms:
| Regulatory Pathway | Function |
|---|---|
| AMPK | Suppresses ATP-consuming processes, enhances glycolysis and autophagy |
| mTOR inhibition | Halts protein synthesis, conserving ATP for essential functions |
| Heat Shock Proteins (HSPs) | Refold or target misfolded proteins for degradation |
| Antioxidant Systems | Detoxify ROS to limit oxidative damage (e.g., glutathione cycle, SOD, catalase) |
| NF-κB / Inflammasome Signaling | Primes inflammatory responses for damage containment and immune recruitment |
| Autophagy / Mitophagy | Degrades damaged organelles to recycle substrates and reduce burden |
These pathways function as metabolic firebreaks—transient programs to prevent irrecoverable failure of core systems. However, they are energetically expensive and often operate under conditions of diminished mitochondrial output.
Energetic Profile
While ATP hydrolysis persists to support containment, mitochondrial ATP regeneration remains impaired. The intracellular ATP pool shrinks, and the accumulation of ADP and inorganic phosphate (Pi) shifts the thermodynamics of ATP hydrolysis:
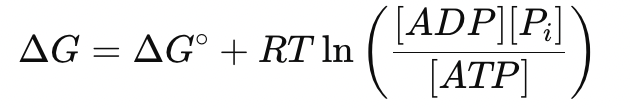
- ΔG becomes less negative, reducing the energy available per hydrolysis event
- Efficiency per ATP molecule declines, increasing energy waste and furthering redox strain
- Ion pumps (e.g., Na⁺/K⁺-ATPase) function suboptimally, compromising excitability and transport
- Membrane repair and emergency transport consume ATP at a rate that exceeds replenishment
Thus, despite maximal effort, the system remains in a state of bioenergetic incoherence: high expenditure with diminishing return.
Summary: The Reaction phase represents a reactive buffering zone, not a solution. Mitochondrial output is insufficient, yet the cell invests heavily in maintaining minimal structure and preventing total collapse. This phase buys time—delaying systemic breakdown and setting the stage for either irreversible failure or structural adaptation.
Adaptation – ATP Regeneration
Adaptation marks the turning point where the system shifts from crisis containment to structural and metabolic reprogramming. While Reaction buys time, Adaptation builds resilience. This phase is not merely recovery—it is reconfiguration. The system does not return to a previous state; instead, it re-establishes coherence under altered conditions.
Mitochondria lie at the center of this transition. As the primary site of ATP regeneration, they must restore oxidative capacity to re-establish negative free energy gradients (ΔG), repolarize membrane potential (Δψ), and stabilize redox dynamics (NAD⁺/NADH). Early adaptation often begins even under partial recovery, but full systemic adaptation requires functional mitochondrial renewal.
Dual Energy Pathways:
1.Mitochondrial Oxidative Phosphorylation (OXPHOS)
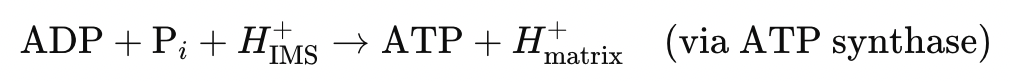
The primary long-term mechanism of ATP regeneration, OXPHOS is reinstated through:
- Electron Transport Chain (ETC) – Complexes I–IV regenerate Δψ and ΔpH
- O₂ availability – Required as the terminal electron acceptor at Complex IV
- Proton Motive Force (PMF) – Drives ATP synthase and powers electrochemical work
- Restored coupling efficiency – Allows ΔG of ATP hydrolysis to return to strongly negative values (~–55 kJ/mol)
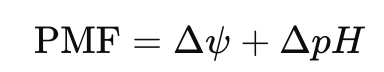
2. Glycolytic ATP Production (Anaerobic Backup)
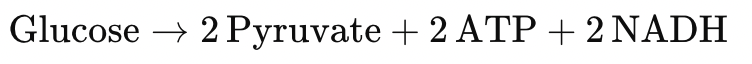
- Less efficient (2 ATP per glucose), but oxygen-independent
- Activated during early Adaptation or when OXPHOS is incomplete
- Regulated by AMPK, HIF-1α, and PFKFB3
- Provides transitional energetic support, especially under hypoxia
While glycolysis supports survival, only mitochondrial OXPHOS can sustain energetic coherence and restore high-efficiency thermodynamic regulation.
Core Mechanisms of Mitochondrial Adaptation:
| Process | Function |
|---|---|
| Mitochondrial Biogenesis | Coordinated by PGC-1α, NRF1, TFAM; increases mitochondrial number and capacity |
| ETC Repair and Resynthesis | Damaged complexes are degraded via mitophagy and rebuilt to restore electron flow |
| NAD⁺/NADH Rebalancing | Enables forward flux through the citric acid cycle and supports complex I function |
| Selective Mitophagy | Removes dysfunctional mitochondria to prevent ROS propagation and energy waste |
| Membrane Potential Restoration | Reestablishes Δψ and ΔpH, key to ATP synthesis and metabolic feedback loops |
Adaptation is also epigenetically encoded: metabolic history influences chromatin accessibility, gene expression (e.g., nuclear-encoded mitochondrial genes), and long-term regulatory setpoints.
Thermodynamic Markers of Successful Adaptation:
- ATP regeneration ≥ ATP hydrolysis
- ΔG (ATP hydrolysis) becomes more negative → restored energy efficiency
- ROS levels normalize → antioxidant systems regain control
- Redox balance (NAD⁺/NADH) stabilizes
- Δψ and ΔpH recover → signaling, ion transport, and biosynthesis resume
- Entropy production slows, while entropy export mechanisms (e.g., heat, metabolic waste, autophagy) re-engage
This phase reestablishes the biophysical gradients that underlie all cellular order, allowing structure to be rebuilt under energetically sustainable conditions.
Summary: Adaptation is the re-integration of the mitochondrial network into the regulatory architecture of the cell. It enables the cell to re-couple energy flow to structural renewal and prepares the system for long-term function under new constraints. By restoring mitochondrial gradients, repairing damage, and resynchronizing energy usage with demand, Adaptation sets the foundation for Refined Homeostasis.
Refined Homeostasis
Refined Homeostasis marks the culmination of adaptive reorganization. Unlike classical homeostasis, which implies a return to a static setpoint, this phase represents a newly established dynamic equilibrium—one shaped by prior stress, encoded through structural, energetic, and regulatory modifications.
At its center lies the reintegrated mitochondrion. Restored mitochondrial function reestablishes the internal thermodynamic engine required to sustain biological order. Proton gradients (Δψ, ΔpH) are stabilized, oxidative phosphorylation resumes at full capacity, and ATP regeneration is once again tightly coupled to system demand.
But the system does not simply return to its pre-disruption configuration. Instead, it adopts a new bioenergetic baseline—one that reflects the stress history, environmental conditions, and internal adaptations of the cell.
Energetic Realignment:
- ATP synthesis and hydrolysis are re-coupled in a closed-loop system
- ΔG for ATP hydrolysis becomes stably negative (≈ –55 to –60 kJ/mol), restoring high energy availability
- Δψ and ΔpH gradients are actively maintained across the mitochondrial membrane
- Redox balance (NAD⁺/NADH) is normalized, enabling efficient flow through the citric acid cycle and ETC
- Entropy production slows, while entropy export mechanisms (e.g., heat dissipation, autophagy, excretion) operate at optimized rates
Energetically, the cell regains coherence not just by resuming energy production, but by tuning that production to match a reorganized demand profile.
Structural and Functional Stability:
- Damaged mitochondria and proteins have been cleared via selective autophagy and proteostasis
- Mitochondrial-ER contact sites (e.g., MAMs) are re-established, restoring cross-organelle communication
- Cytoskeletal networks and membranes are rebuilt for optimized transport and signal propagation
- Organelle distribution and function (e.g., localized ATP zones in neurons, calcium buffering near ER) reflect reallocation of energetic priorities
This structural realignment reflects a cell-wide integration of mitochondrial recovery, stabilizing not just function but spatial coordination.
Regulatory Encoding and Rhythmic Resynchronization:
- Epigenetic modifications (e.g., histone acetylation, DNA methylation) encode stress memory and re-tune gene expression
- Mitochondria–nucleus crosstalk updates transcriptional programs based on redox and energetic cues
- Oscillatory systems—circadian rhythms, redox cycles, metabolic clocks—are resynchronized with mitochondrial output
- Stress response pathways (AMPK, HIF-1α) are deactivated but remain primed for future activation
Integrated Coherence
Together, these updates form a regulatory memory—allowing the cell to respond more efficiently to similar challenges in the future.
In Refined Homeostasis, mitochondria are no longer just recovered—they are redefined. Their protein composition, spatial distribution, and metabolic behavior now reflect both historical experience and future resilience. They serve as:
- Entropy converters – transforming external substrates into sustained internal order
- Energetic sensors – modulating systemic activity based on real-time flux
- Memory nodes – encoding prior disruption in their structure and epigenetic outputs
- Rhythmic anchors – synchronizing cell-wide cycles to energy availability
Summary:
Refined Homeostasis represents a new organizational attractor, emerging from the system’s interaction with stress. Through the restoration and reprogramming of mitochondrial function, the cell reclaims its ability to operate far from thermodynamic equilibrium. Not as a static state—but as a dynamically coherent regime, optimized for adaptive efficiency, ready for the next inflection in entropy flow.
References
Friston, K. (2010).
The free-energy principle: a unified brain theory?
Nature Reviews Neuroscience, 11(2), 127–138.
https://doi.org/10.1038/nrn2787
Voorsluijs, V., Avanzini, F., Falasco, G., Esposito, M., & Skupin, A. (2023).
Nonequilibrium calcium dynamics optimizes the energetic efficiency of mitochondrial metabolism.
arXiv:2303.08822
Viollet, B. (2019).
The Energy Sensor AMPK: Adaptations to Exercise, Nutritional and Hormonal Signals.
arXiv:1911.02345
Kapitanov, G. I., Ayati, B. P., & Martin, J. A. (2016).
Modeling the Effect of Blunt Impact on Mitochondrial Dysfunction in Cartilage.
arXiv:1612.03859
Arias-Hernández, L. A., Paez-Hernández, R. T., & Angulo-Brown, F. (2007).
A first-order irreversible thermodynamic approach to a simple energy converter.
arXiv:0709.0321
Boersch, M. (2010).
Targeting cytochrome C oxidase in mitochondria with Pt(II)-porphyrins for Photodynamic Therapy.
arXiv:1002.1023
Zhou, J., Dhakal, K., & Yi, J. (2016).
Mitochondrial Ca²⁺ uptake in skeletal muscle health and disease.
arXiv:1607.08507
Tewari, S. G., Dash, R. K., Beard, D. A., & Bazil, J. N. (2012).
A Biophysical Model of the Mitochondrial ATP-Mg/Pi Carrier.
arXiv:1206.7053
Manoj, K. M. (2017).
Mitochondrial oxidative phosphorylation: Debunking the concepts of electron transport chain, proton pumps, chemiosmosis and rotary ATP synthesis.
arXiv:1703.05826
